Coffee Grind Settling Calculator
Quick Start
This App uses Stokes’ law to estimate the rate at which coffee grinds (including the fines) will settle in your brewing vessel. The amazing Boycott effect which greatly increases the settling rate in inclined tubes is added as a bonus.
Credits
The app is another created while working with and inspired by the Barista Hustle team.
Coffee Grind Settling
Getting your coffee particles to settle
The graphs show your main particles (P) settling from the top and from half-way down (P½), plus the Fines (F and F½). By changing the tmax slider you can look at longer or shorter brew times.
The science is described below, but there are just three things you need to know to ensure you have good settling:
- Larger particles settle much faster. If you halve the size of the particle, it falls 4x slower. A fines particle that is 10x smaller will settle 100x slower, i.e. it won't settle in any reasonable time.
- Denser particle settle faster. And light roasts are denser than medium roasts which are denser than dark roasts (because roasting puffs up the beans). As a rule of thumb, densities of 1.05 for Dark, 1.1 for Medium and 1.2 for Light give 2x faster settling for each step. Light roasts are much easier to settle.
- OK, so there are plenty of other variables such as type and age of the coffee, but the effects of size and density will dominate.
The science: Stokes law
When you have particles of diameter D of density ρp in a liquid of viscosity η and density ρl and a gravitational force g then the particles will fall at a velocity v and cover a distance h (the depth of your brewing system) in time t. The formula is the Stokes equation:
`v=0.545(ρ_p-ρ_l)(gD^2)/η`
`h=tv`
So far, we have assumed that the particles don't interfere with themselves. A simple, but effective approximation from Richardson and Zaki tells us that the velocity is reduced by a factor of (1-φ/100)5.65 where φ is the % coffee particles. Clearly this means that as particles start to settle and get locally more concentrated, the rate will reduce. This app is just to give a general idea of the effects in the early stages of settling.
Although φ for a typical immersion brew is only 6%, when you go to a cezve at 10% or espresso-style at 30%, things slow down quite a lot.
The Boycott effect
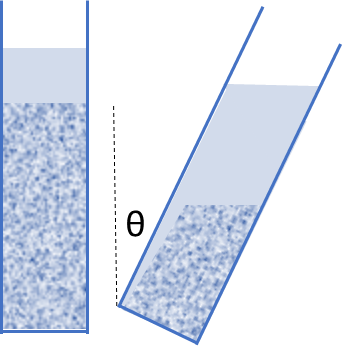 In the 1920's, Boycott noticed that red blood cells settled much faster in a tilted tube than in a vertical one. The effect is real. During the settling, a thin clear layer appears between the particles and the upper sloped wall. There are various explanations for the effect. One says that the particles have a smaller distance to fall till they reach the wall and can then slide down the wall. I prefer the equivalent view that the clear layer is a back channel for solvent displaced by the falling particles to escape - something not possible in a vertical tube.
In the 1920's, Boycott noticed that red blood cells settled much faster in a tilted tube than in a vertical one. The effect is real. During the settling, a thin clear layer appears between the particles and the upper sloped wall. There are various explanations for the effect. One says that the particles have a smaller distance to fall till they reach the wall and can then slide down the wall. I prefer the equivalent view that the clear layer is a back channel for solvent displaced by the falling particles to escape - something not possible in a vertical tube.
The theory is simple. Where H is the height of the particle column, b is the width of the tube (well, the theory assumes parallel plates) and the tilt angle is θ, defined as 0 for a vertical tube, if we start with the velocity v calculated above then the new Boycott velocity, VB is given by:
`v_B = v(1+H/bsinθ)`
And if you don't believe the theory, see it in action. This wonderful YouTube video on the Boycott effect is very convincing.

